Hich Best Describes a Beta Particle
See answers 2 Best Answer. The beta particle like the electron has a very small mass compared to the proton or neutron.
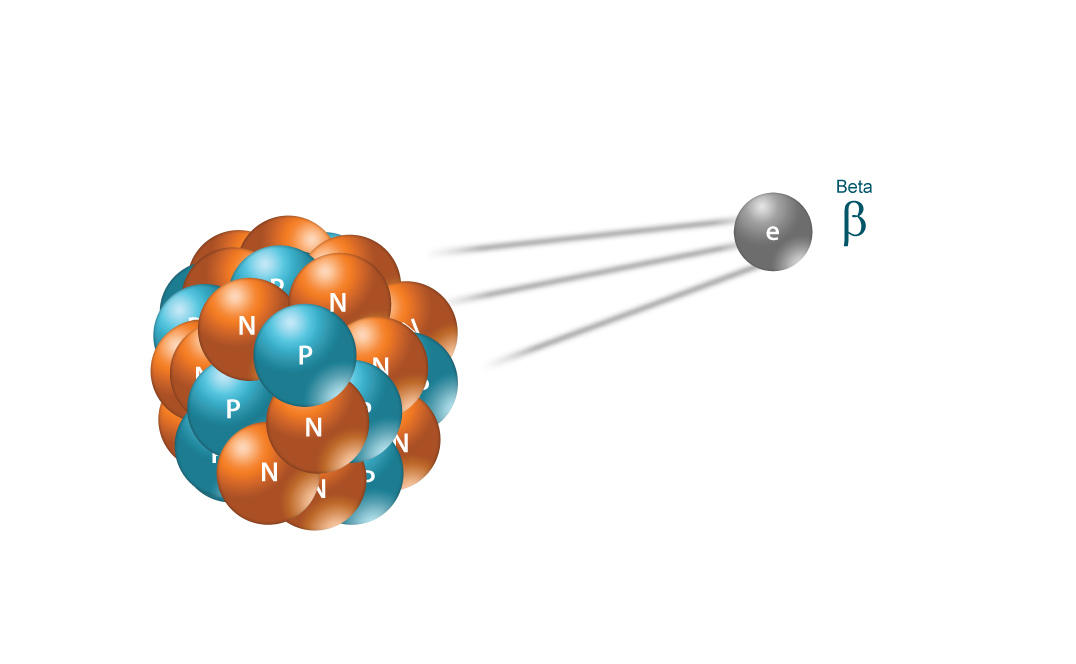
A beta particle _-1colorwhiteaa0beta is simply a high-speed electronWhen a radioactive nuclide undergoes beta minus decay a neutron located inside its nucleus is being.

. Moves much slower. A beta β particle is described by which of the following. It has a charge of -1 and very little mass only 1 1840 as big as a proton.
The e shows that the particle is an electron. What best describes a beta particle. The beta particle is a high-speed electron or positron released from a degenerating radioactive nucleus.
The mass numbers are the same. It is often called a high energy electron because it is very fast moving. There are two types of beta decay.
Which answer best describes an alpha particle. Strontium-90 90Sr undergoes beta minus decay so your goal here will be to use what you know about the emission of a beta particle to figure out the resulting nuclide. A β particle is the antiparticle to the electron.
A beta particle comes from the nucleus of an atom. A negative charge and no mass C. 4An alpha particle has less mass than a beta particle.
The answer is a positively charged nucleus with two protons and two neutrons. A beta particle is emitted from the nucleus of an atom during radioactive decay. See answer 1 Best Answer.
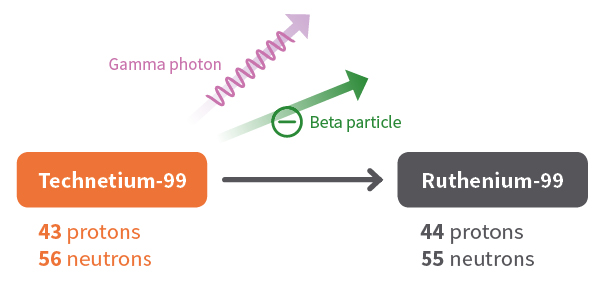
Similarly if a neutron is converted to a proton it is known as β- decay. During beta decay the proton in the nucleus is transformed into a neutron and vice versa. What is particle X.
If a proton is converted to neutron it is known as β decay. Beta particles are either high energy electrons or high energy positrons. A positive charge and a mass number of 2 D.
Which of the following describes radioactive decay by beta particle emission. Which of the following statements best describes why a large nucleus is more likely to undergo radioactive decay. A beta particle also called beta ray or beta radiation symbol β is a high-energy high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decayThere are two forms of beta decay β decay and β decay which produce electrons and positrons respectively.
March 24 2022 Beta particles are not radioactive but they can cause other damage. Asked Sep 13 2016 in Environmental Atmospheric Sciences by ANerdWW. A positive charge and a mass number of 4 12.
Beta particles with an energy of 05 MeV have a range of about one. Aabsorb electrons Babsorb protons Cdecay Doxidize 2The nucleus of a radium-226 atom is unstable which causes the nucleus to spontaneously Aalpha particle beta particle gamma radiation Bgamma radiation alpha particle beta particle Cpositron alpha particle neutron Dneutron positron alpha particle. 1a ground-state electron 2a stable nucleus 3an excited electron 4an unstable nucleus 10A beta particle may be.
An alpha particle B. Beta particles are named after the second letter in Greek alphabet the letter β. These are emitted in the decay of various radioactive nuclei such as Potassium 40.
Select the correct answer below. A beta particle also written as β-particle is the same as an electron. Mass of 0 amu.
232 90 Th 228 88 Ra X The letter X in the equation represents A. Up to 24 cash back 11. A β particle is another term for an electron orbiting a stable nucleus.
1alpha particle 2beta particle 3neutron 4proton 9Which particle has the least mass. A recommendation that is to be applied when practical is indicated by the use of which of these terms. What is a Beta Particle.
Given the nuclear equation. Which of the following describes a Beta particle. A beta particle C.
The alpha particle has twice the electric charge of the beta particle but deflects less than the beta in a magnetic field because it _____. A gamma ray is best described as having A. 8Which statement describes the relative masses of two different particles.
Beta decay is a radioactive decay in which a beta ray is emitted from an atomic nucleus. Beta particles are also denoted as β particles. A β particle is a high-energy electron.
A The atomic number of the daughter isotope is one more than the parent. B The daughter isotope has an atomic number one less than the parent and a mass number two. Being medium-energy and low mass beta.
The electron however occupies regions outside the nucleus of an atom. A β particle is the antimatter equivalent of a proton. The beta particle is a form of ionizing radiation related to other common forms of radiation alpha particles and gamma rays.
Its mass is 11836 that of the proton mass or 11838 that of the neutron mass. No electric charge and no mass B. Which of the following is the unit for measuring the activity of a radionuclide.

No comments for "Hich Best Describes a Beta Particle"
Post a Comment